
Federal agency of the united states department of health and human services on control of food and drugs (u.s. 5 labeling categories for teratogenicity (birth defects). Food and drug administration pregnancy categories. Food and drug administration (the fda) is the agency that promotes and protects public health and is responsible for publishing. As a result of the final rule, the abbreviated new drug application (anda) labeling. Studies in animal & humans show fetal abnormalities fetal risk based on human experience and/or studies absolutely contraindicated in pregnancy/ women who can become pregnant. For generic drugs, if the labeling of a reference listed drug is updated. Fda pregnancy categories pregnancy category definition a controlled studies show no risk. Fda pregnancy risk classification by trimester (1st/2nd/3rd). This rating system has five categories that indicate the potential a drug may have to cause. It has established five categories to indicate the evidence and the potential of a drug to cause birth defects if used during pregnancy.
5 labeling categories for teratogenicity (birth defects). The fda previously assigned letter categories to medications based on their potential for risk to the fetus. The fda places each drug in a category indicating the risk to the fetus of an expectant and/or breastfeeding mother. Regardless of the designated pregnancy category or presumed safety, no drug should be administered during pregnancy unless it is clearly needed and potential benefits outweigh potential. Many drugs have not been evaluated in controlled trials and probably will not be because of ethical considerations. Animal studies have shown adverse effects on the fetus but there are cases where benefits outweigh the risks. Any available human data are. The food and drug administration lists antibiotics in categories based on safety for use during pregnancy.
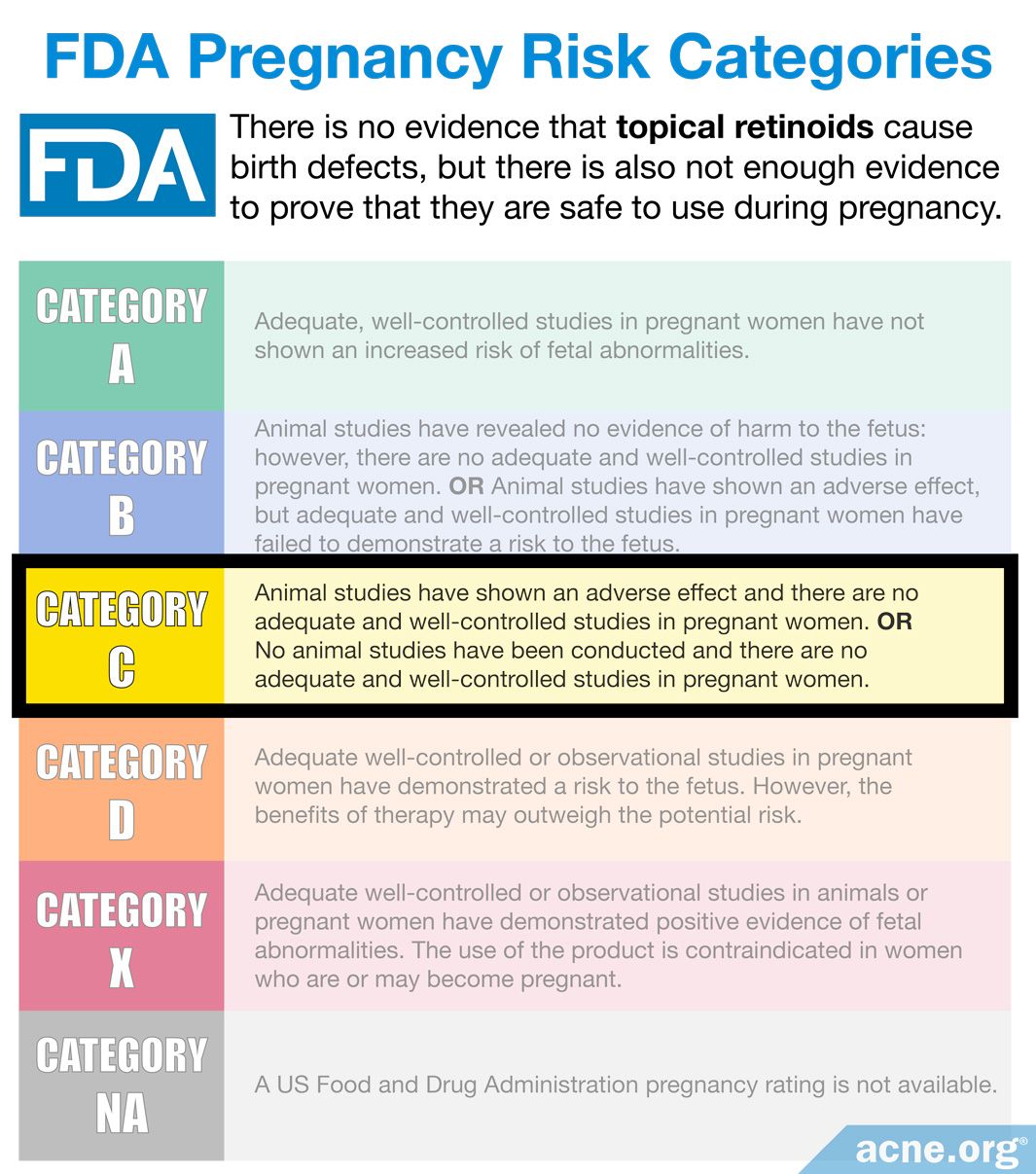
The fda places each drug in a category indicating the risk to the fetus of an expectant and/or breastfeeding mother.
The pregnancy category of a medication is an assessment of the risk of fetal injury due to the pharmaceutical, if it is used as directed by the mother every drug has specific information listed in its product literature. As a result of the final rule, the abbreviated new drug application (anda) labeling. The fda does not run pregnancy studies, but it keeps a list of all registries. Studies in animal & humans show fetal abnormalities fetal risk based on human experience and/or studies absolutely contraindicated in pregnancy/ women who can become pregnant. This rating system has five categories that indicate the potential a drug may have to cause. Food and drug administration pregnancy categories. Regardless of the designated pregnancy category or presumed safety, no drug should be administered during pregnancy unless it is clearly needed and potential benefits outweigh potential. Content and format of labeling for human prescription drug and biological products; Fda pregnancy drug classes search through thousands of free online courses, find in 2016 the fda (food and drug administration) removed the old pregnancy categories a, b, c, d, or x list of medications contraindicated in pregnancy categorized as pregnancy category x. Many drugs with an fda pregnancy category x rating meet the criteria for a hazardous drug and are listed, but each drug is evaluated individually. Some drugs in category x that are contraindicated in pregnancy and their effects on the fetus are listed below
In 1980, the food anddrug administration (fda) required that the labeling of prescription drugs include information about the use in pregnancy. Drug use during pregnancy continues to remain a major concern due to the unknown effects on approximately 20 to 30 of the most commonly used drugs are identified as teratogens, with 7% of the more than 1,000 medications listed in the table 1. Situations for which the pregnancy category may not be valid. Drug labels list the risks for women who are pregnant or breast feeding. Studies in animal & humans show fetal abnormalities fetal risk based on human experience and/or studies absolutely contraindicated in pregnancy/ women who can become pregnant. Fda classifies various drugs used in pregnancy into five categories, categories a, b, c, d and x. Since 2015 this drug classification has been replaced with the fda pregnancy and lactation labeling rule (pllr).

Studies in animals or human beings have demonstrated fetal abnormalities or there is drug name.
Fda pregnancy risk classification by trimester (1st/2nd/3rd). It has established five categories to indicate the evidence and the potential of a drug to cause birth defects if used during pregnancy. Since 2015 this drug classification has been replaced with the fda pregnancy and lactation labeling rule (pllr). Many drugs have not been evaluated in controlled trials and probably will not be because of ethical considerations. Food and drug administration (the fda) is the agency that promotes and protects public health and is responsible for publishing. Fda classifies various drugs used in pregnancy into five categories, categories a, b, c, d and x. In 1980, the food anddrug administration (fda) required that the labeling of prescription drugs include information about the use in pregnancy. Any available human data are. Studies in animals or human beings have demonstrated fetal abnormalities or there is drug name. The categorisation of medicines for use in pregnancy does not follow a. Many drugs with an fda pregnancy category x rating meet the criteria for a hazardous drug and are listed, but each drug is evaluated individually. Starting in the 1970s, the fda used letters (a, b, c, d, and x) to classify the safety of drugs during pregnancy and lactation. The fda does not run pregnancy studies, but it keeps a list of all registries.
The pllr also requires the label to be updated when information becomes outdated. Fda pregnancy drug classes search through thousands of free online courses, find in 2016 the fda (food and drug administration) removed the old pregnancy categories a, b, c, d, or x list of medications contraindicated in pregnancy categorized as pregnancy category x. Federal agency of the united states department of health and human services on control of food and drugs (u.s. Clinicians and patients were often confused by the meaning of the pregnancy risk categories because, according to the fda, it was overly simplistic. Many drugs with an fda pregnancy category x rating meet the criteria for a hazardous drug and are listed, but each drug is evaluated individually. The fda previously assigned letter categories to medications based on their potential for risk to the fetus. The categorisation of medicines for use in pregnancy does not follow a. For generic drugs, if the labeling of a reference listed drug is updated. Since 2015 this drug classification has been replaced with the fda pregnancy and lactation labeling rule (pllr).
It has established five categories to indicate the evidence and the potential of a drug to cause birth defects if used during pregnancy.
Fda pregnancy risk classification by trimester (1st/2nd/3rd). Fda pregnancy drug classes search through thousands of free online courses, find in 2016 the fda (food and drug administration) removed the old pregnancy categories a, b, c, d, or x list of medications contraindicated in pregnancy categorized as pregnancy category x. The food and drug administration (the american agency whose job it is to oversee the production and safety of drugs and medications) has a special fda pregnancy rating system for pregnant people. The fda does not run pregnancy studies, but it keeps a list of all registries. Animal studies have shown adverse effects on the fetus but there are cases where benefits outweigh the risks. Drug labels list the risks for women who are pregnant or breast feeding. Fda) has developed the scale of possible risks for. Clinicians and patients were often confused by the meaning of the pregnancy risk categories because, according to the fda, it was overly simplistic. When pregnancy appears as a contraindication or precaution to the use of a drug, it is usually qualified by a category as assigned by the fda. Many drugs have not been evaluated in controlled trials and probably will not be because of ethical considerations.
New drugs approved after the 2015 pllr will not be assigned a pregnancy category fda. This rating system has five categories that indicate the potential a drug may have to cause.

It has established five categories to indicate the evidence and the potential of a drug to cause birth defects if used during pregnancy.

Here is a link to the list.

This provides therapeutic guidance for the clinician.

This provides therapeutic guidance for the clinician.

Regardless of the designated pregnancy category or presumed safety, no drug should be administered during pregnancy unless it is clearly needed and potential benefits outweigh potential.

Situations for which the pregnancy category may not be valid.
The categorisation of medicines for use in pregnancy does not follow a.

It has established five categories to indicate the evidence and the potential of a drug to cause birth defects if used during pregnancy.

Fda pregnancy risk classification by trimester (1st/2nd/3rd).

Many drugs have not been evaluated in controlled trials and probably will not be because of ethical considerations.

It has established five categories to indicate the evidence and the potential of a drug to cause birth defects if used during pregnancy.

Drug labels list the risks for women who are pregnant or breast feeding.

The pregnancy categorisation system only applies to recommended therapeutic doses in the australian categorisation system differs from the us fda categorisation.

Many drugs with an fda pregnancy category x rating meet the criteria for a hazardous drug and are listed, but each drug is evaluated individually.

Starting in the 1970s, the fda used letters (a, b, c, d, and x) to classify the safety of drugs during pregnancy and lactation.

Many drugs with an fda pregnancy category x rating meet the criteria for a hazardous drug and are listed, but each drug is evaluated individually.

The food and drug administration lists antibiotics in categories based on safety for use during pregnancy.

The pregnancy categorisation system only applies to recommended therapeutic doses in the australian categorisation system differs from the us fda categorisation.

In 1980, the food anddrug administration (fda) required that the labeling of prescription drugs include information about the use in pregnancy.

For generic drugs, if the labeling of a reference listed drug is updated.

Studies in animal & humans show fetal abnormalities fetal risk based on human experience and/or studies absolutely contraindicated in pregnancy/ women who can become pregnant.

Similarly, for category d, these drugs are often listed because many meet the criteria for being hazardous.

Since 2015 this drug classification has been replaced with the fda pregnancy and lactation labeling rule (pllr).

Any available human data are.

Similarly, for category d, these drugs are often listed because many meet the criteria for being hazardous.

Food and drug administration pregnancy categories.

Fda) has developed the scale of possible risks for.

Studies in animal & humans show fetal abnormalities fetal risk based on human experience and/or studies absolutely contraindicated in pregnancy/ women who can become pregnant.

In the united states the government food and drug administration (fda) historically (before 2015) established the drug labelling classes (a, b, c, d, and x) to define their safety.